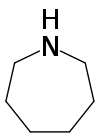
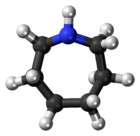
Azepane
Chemical compound
Azepane is the organic compound with the formula (CH2)6NH. It is a colorless liquid. A cyclic secondary amine, it is a precursor to several drugs and pesticides. It is produced by partial hydrogenolysis of hexamethylene diamine.[2]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Azepane | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C6H13N | |||
| Molar mass | 99.177 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Density | 0.88 g/cm3[1] | ||
| Melting point | −37 °C (−35 °F; 236 K) | ||
| Boiling point | 138 °C (280 °F; 411 K)[1] (749 mmHg) | ||
| Hazards | |||
| Flash point | 30 °C (86 °F; 303 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Like many amines, it reacts with carbon dioxide.[3]
Azepane-containing drugs
A variety of pharmaceutical drugs contain an azepane ring including bazedoxifene, cetiedil, glisoxepide, mecillinam, nabazenil, setastine, and tolazamide, among others.
See also
References
- ^ 1.0 1.1 "Hexamethyleneimine".
- ^ Karsten Eller; Erhard Henkes; Roland Rossbacher; Hartmut Höke (2005). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001. ISBN 3527306730.
- ^ Sanz-Pérez, E. S.; Arencibia, A.; Sanz, R.; Calleja, G. (2016). "New developments on carbon dioxide capture using amine-impregnated silicas". Adsorption. 22 (4): 366–375. doi:10.1007/s10450-015-9740-2. S2CID 100692983.