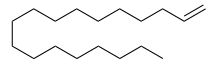
1-Octadecene
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Octadec-1-ene | |
| Other names
alpha-Octadecene; Octadecylene; alpha-Olefin C18; n-1-Octadecene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H36 | |
| Molar mass | 252.486 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.789 g/mL[1] |
| Melting point | 14 to 16 °C (57 to 61 °F; 287 to 289 K)[1] 17 to 18 °C[2] |
| Boiling point | 315 °C (599 °F; 588 K)[2] |
| Insoluble[2] | |
| Hazards | |
| NFPA 704 (fire diamond) | <imagemap>
File:NFPA 704.svg|80px|alt=NFPA 704 four-colored diamond poly 150 150 300 300 150 450 0 300 Health 1: Exposure would cause irritation but only minor residual injury. E.g. turpentine poly 300 0 450 150 300 300 150 150 Flammability 1: Must be pre-heated before ignition can occur. Flash point over 93 °C (200 °F). E.g. canola oil poly 450 150 600 300 450 450 300 300 Instability 0: Normally stable, even under fire exposure conditions, and is not reactive with water. E.g. liquid nitrogen poly 300 300 450 450 300 600 150 450 Special hazards (white): no code desc none </imagemap> |
| Flash point | 155 °C (311 °F; 428 K)[2] |
| 250 °C (482 °F; 523 K)[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1-Octadecene is a long-chain hydrocarbon and an alkene with the molecular formula CH2=CH(CH2)15CH3. It is one of many isomers of octadecene. Classified as an alpha-olefin, 1-octadecene is the longest alkene that is liquid at room temperature.[3][2]
Hydrosilation
Treatment of 1-octadecene with trichlorosilane in the presence of platinum catalysts gives octadecyltrichlorosilane. Octadecene adds to hydrogen-terminated bulk silicon.[4]
See also
References
- ^ 1.0 1.1 1.2 1-Octadecene at Sigma-Aldrich
- ^ 2.0 2.1 2.2 2.3 2.4 1-Octadecene fact sheet ChemicalLand21
- ^ Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke, Hartmut (2000). "Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_227. ISBN 3527306730.
- ^ Linford, Matthew R.; Fenter, Paul; Eisenberger, Peter M.; Chidsey, Christopher E. D. (1995). "Alkyl Monolayers on Silicon Prepared from 1-Alkenes and Hydrogen-Terminated Silicon". Journal of the American Chemical Society. 117 (11): 3145–3155. doi:10.1021/ja00116a019.