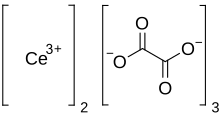
Cerium oxalate
| |

| |
| Names | |
|---|---|
| IUPAC name
Cerium(III) oxalate
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6Ce2O12 | |
| Molar mass | 544.286 g·mol−1 |
| Appearance | White crystals |
| Melting point | Decomposes |
| Slightly soluble | |
| Pharmacology | |
| A04AD02 (WHO) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Corrosive, Irritant, Respiratory irritant, Toxic |
| GHS labelling: | |
   [1] [1]
| |
| Danger[1] | |
| H301, H311, H314, H319, H331, H335, H370[1] | |
| P260, P264, P270, P271, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P308+P313, P332+P313, P403+P233[1] | |
| NFPA 704 (fire diamond) | <imagemap>
File:NFPA 704.svg|80px|alt=NFPA 704 four-colored diamond poly 150 150 300 300 150 450 0 300 Health 3: Short exposure could cause serious temporary or residual injury. E.g. chlorine gas poly 300 0 450 150 300 300 150 150 Flammability 0: Will not burn. E.g. water poly 450 150 600 300 450 450 300 300 Instability 1: Normally stable, but can become unstable at elevated temperatures and pressures. E.g. calcium poly 300 300 450 450 300 600 150 450 Special hazards (white): no code desc none </imagemap> |
| Flash point | 188.8 °C |
| Safety data sheet (SDS) | External SDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cerium(III) oxalate (cerous oxalate) is the inorganic cerium salt of oxalic acid. It is a white crystalline solid with the chemical formula of Ce2(C2O4)3. It could be obtained by the reaction of oxalic acid with cerium(III) chloride.
Uses
Cerium(III) oxalate is used as an antiemetic.[2][3] It has been identified as part of the invisible ink that was used by Stasi operatives during the Cold War.[4]
Toxicity
Cerium(III) oxalate irritates skin and mucous membranes, and is a strong irritant to eyes. If it gets into the eyes, there is a danger of severe eye injury.
Cerium salts increase the blood coagulation rate, and exposure to cerium salts can cause sensitivity to heat.
Oxalates are corrosive to tissue and are powerful irritants. They have a caustic effect on the linings of the digestive tracts and can cause kidney damage.
References
This article needs additional citations for verification. (April 2011) |
- ^ 1.0 1.1 1.2 1.3 "Cerium(III) Oxalate, Anhydrous". American Elements. Retrieved 2019-03-26.
- ^ "KEGG DRUG: Cerium oxalate". KEGG DRUG Database. Retrieved 2019-03-26.
- ^ Milne, G. W. A. (2017-11-01). Drugs: Synonyms and Properties: Synonyms and Properties. Routledge. ISBN 9781351755092.
- ^ "Cold War Invisible Ink Secrets Unlocked". ScienceDaily. 2006-11-08.
- Articles without InChI source
- Articles without EBI source
- Articles without KEGG source
- Short description with empty Wikidata description
- Articles needing additional references from April 2011
- Articles with invalid date parameter in template
- All articles needing additional references
- Cerium(III) compounds
- Oxalates
- Antiemetics