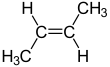
But-2-ene
But-2-ene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism (also known as (E/Z)-isomerism); that is, it exists as two geometric isomers cis-but-2-ene ((Z)-but-2-ene) and trans-but-2-ene ((E)-but-2-ene).
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
But-2-ene | |||
| Other names
β-Butylene
| |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| 1718755 1361341 | |||
| ChEBI |
| ||
| ChemSpider | |||
| EC Number |
| ||
| 25196 1140 1141 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII |
| ||
| |||
| |||
| Properties | |||
| C4H8 | |||
| Molar mass | 56.106 g/mol | ||
| Density | 0.641 g/ml (cis, 3.7 °C)[1] 0.626 g/ml (trans, 0.9 °C)[2] | ||
| Melting point | −138.9 °C (−218.0 °F; 134.2 K) (cis)[1] -105.5 °C (trans)[2] | ||
| Boiling point | 0.8 to 3.7 °C (33.4 to 38.7 °F; 273.9 to 276.8 K) (Z = 3.7 °C)[1] (E = 0.8 °C)[2] | ||
| |||
| Hazards[3] | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H220 | |||
| P210, P377, P381, P403 | |||
| NFPA 704 (fire diamond) | <imagemap>
File:NFPA 704.svg|80px|alt=NFPA 704 four-colored diamond poly 150 150 300 300 150 450 0 300 Health 1: Exposure would cause irritation but only minor residual injury. E.g. turpentine poly 300 0 450 150 300 300 150 150 Flammability 4: Will rapidly or completely vaporize at normal atmospheric pressure and temperature, or is readily dispersed in air and will burn readily. Flash point below 23 °C (73 °F). E.g. propane poly 450 150 600 300 450 450 300 300 Instability 0: Normally stable, even under fire exposure conditions, and is not reactive with water. E.g. liquid nitrogen poly 300 300 450 450 300 600 150 450 Special hazards (white): no code desc none </imagemap> | ||
| Flash point | −72 °C (−98 °F; 201 K)[1][2] | ||
| 325 °C (617 °F; 598 K)[1][2] | |||
| Related compounds | |||
Related butenes
|
1-Butene cis-2-Butene trans-2-Butene Isobutene | ||
Related compounds
|
Butane Butyne | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
It is a petrochemical, produced by the catalytic cracking of crude oil or the dimerization of ethylene. Its main uses are in the production of gasoline (petrol) and butadiene,[4] although some but-2-ene is also used to produce the solvent butanone via hydration reaction to butan-2-ol followed by oxidation.
The two isomers are extremely difficult to separate by distillation because of the proximity of their boiling points (~4 °C for cis and ~1 °C for trans[5]). However, separation is unnecessary in most industrial settings, as both isomers behave similarly in most of the desired reactions. A typical industrial but-2-ene mixture is 70% (Z)-but-2-ene (cis-isomer) and 30% (E)-but-2-ene (trans-isomer). Butane and but-1-ene are common impurities, present at 1% or more in industrial mixtures, which also contain smaller amounts of isobutene, butadiene and butyne.[4]
References
- ^ 1.0 1.1 1.2 1.3 1.4 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ 2.0 2.1 2.2 2.3 2.4 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ cis-2-Butene, International Chemical Safety Card 0397, Geneva: International Programme on Chemical Safety, March 1996. trans-2-Butene, International Chemical Safety Card 0398, Geneva: International Programme on Chemical Safety, March 1996.
- ^ 4.0 4.1 2-Butene (PDF), SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, February 1995.
- ^ Chemical Safety Information from Intergovernmental Organizations Archived December 9, 2009, at the Wayback Machine