From English Wikipedia @ Freddythechick
Chemical element with atomic number 11 (Na)
Sodium, 11 Na Appearance silvery white metallic
Atomic number (Z ) 11 Group group 1: hydrogen and alkali metals Period period 3 Block s-block Electron configuration [Ne ] 3s1 Electrons per shell 2, 8, 1 Phase at STP solid Melting point 370.944 K (97.794 °C, 208.029 °F) Boiling point 1156.090 K (882.940 °C, 1621.292 °F) Density (at 20° C) 0.9688 g/cm3 [3] when liquid (at m.p. ) 0.927 g/cm3 Critical point 2573 K, 35 MPa (extrapolated) Heat of fusion 2.60 kJ/mol Heat of vaporization 97.42 kJ/mol Molar heat capacity 28.230 J/(mol·K) Vapor pressure
P (Pa)
1
10
100
1 k
10 k
100 k
at T (K)
554
617
697
802
946
1153
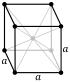
Oxidation states −1, 0,[4] +1 basic oxide) Electronegativity Pauling scale: 0.93 Ionization energies 1st: 495.8 kJ/mol 2nd: 4562 kJ/mol 3rd: 6910.3 kJ/mol (more ) Atomic radius empirical: 186 pm Covalent radius 166±9 pm Van der Waals radius 227 pm Spectral lines of sodiumNatural occurrence primordial Crystal structure body-centered cubic (bcc) (cI2 ) Lattice constant a = 428.74 pm (at 20 °C)[3] Thermal expansion × 10−6 [3] Thermal conductivity 142 W/(m⋅K) Electrical resistivity 47.7 nΩ⋅m (at 20 °C) Magnetic ordering paramagnetic [5] Molar magnetic susceptibility × 10−6 3 /mol (298 K)[6] Young's modulus 10 GPa Shear modulus 3.3 GPa Bulk modulus 6.3 GPa Speed of sound thin rod 3200 m/s (at 20 °C) Mohs hardness 0.5 Brinell hardness 0.69 MPa CAS Number 7440-23-5 Discovery and first isolationHumphry Davy (1807)Symbol "Na": from New Latin natrium Natron natron '
Category: Sodium references
child table, as reused in {IB-Na}
References These references will appear in the article, but this list appears only on this page.
^ "Standard Atomic Weights: Sodium" . CIAAW . 2005.^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)" . Pure and Applied Chemistry . doi :10.1515/pac-2019-0603 . ISSN 1365-3075 . ^ 3.0 3.1 3.2 Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements . Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9 ^ The compound NaCl has been shown in experiments to exists in several unusual stoichiometries under high pressure, including Na3 Cl in which contains a layer of sodium(0) atoms; see Zhang, W.; Oganov, A. R.; Goncharov, A. F.; Zhu, Q.; Boulfelfel, S. E.; Lyakhov, A. O.; Stavrou, E.; Somayazulu, M.; Prakapenka, V. B.; Konôpková, Z. (2013). "Unexpected Stable Stoichiometries of Sodium Chlorides". Science . 342 (6165): 1502–1505. arXiv :1310.7674 Bibcode :2013Sci...342.1502Z . doi :10.1126/science.1244989 . PMID 24357316 . S2CID 15298372 .
^ Magnetic susceptibility of the elements and inorganic compounds , in Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5 ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics . Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4 ^ 7.0 7.1 Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF) . Chinese Physics C . 45 (3): 030001. doi :10.1088/1674-1137/abddae . One of these is a named reference . It may be cited in the containing article as
<ref name="CIAAW2013" /> for the source Atomic weights of the elements 2013 (from subtemplates used by {{ Infobox element }} ) Lua error in mw.title.lua at line 229: too many expensive function calls.