From English Wikipedia @ Freddythechick
Chemical element with atomic number 74 (W)
Tungsten, 74 W Pronunciation (TUNG -stən Alternative name Wolfram, pronounced: (WUUL -frəm Allotropes α-tungsten (common), β-tungsten Appearance Grayish white, lustrous
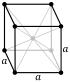
Atomic number (Z ) 74 Group group 6 Period period 6 Block d-block Electron configuration [Xe ] 4f14 5d4 6s2 [3] Electrons per shell 2, 8, 18, 32, 12, 2 Phase at STP solid Melting point 3695 K (3422 °C, 6192 °F) Boiling point 6203 K (5930 °C, 10706 °F) Density (at 20° C) 19.254 g/cm3 [4] when liquid (at m.p. ) 17.6 g/cm3 Heat of fusion 52.31 kJ/mol [5] [6] Heat of vaporization 774 kJ/mol Molar heat capacity 24.27 J/(mol·K) Vapor pressure
P (Pa)
1
10
100
1 k
10 k
100 k
at T (K)
3477
3773
4137
4579
5127
5823
Oxidation states −4, −2, −1, 0, +1, +2, +3, +4 +6 acidic oxide) Electronegativity Pauling scale: 2.36 Ionization energies 1st: 770 kJ/mol 2nd: 1700 kJ/mol Atomic radius empirical: 139 pm Covalent radius 162±7 pm Spectral lines of tungstenNatural occurrence primordial Crystal structure body-centered cubic (bcc) (cI2 ) Lattice constant a = 316.52 pm (at 20 °C)[4] Thermal expansion × 10−6 [4] Thermal conductivity 173 W/(m⋅K) Electrical resistivity 52.8 nΩ⋅m (at 20 °C) Magnetic ordering paramagnetic [7] Molar magnetic susceptibility × 10−6 3 /mol (298 K)[8] Young's modulus 411 GPa Shear modulus 161 GPa Bulk modulus 310 GPa Speed of sound thin rod 4620 m/s (at r.t. ) (annealed) Poisson ratio 0.28 Mohs hardness 7.5 Vickers hardness 3430–4600 MPa Brinell hardness 2000–4000 MPa CAS Number 7440-33-7 Discovery and first isolationJuan José Elhuyar and Fausto Elhuyar [9] Named by Torbern Bergman (1781) Symbol "W": from Wolfram , originally from Middle High German wolf-rahm 'wolf's foam' describing the mineral wolframite [10]
Category: Tungsten references
child table, as reused in {IB-W}
References These references will appear in the article, but this list appears only on this page.
^ "Standard Atomic Weights: Tungsten" . CIAAW . 1991.^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)" . Pure and Applied Chemistry . doi :10.1515/pac-2019-0603 . ISSN 1365-3075 . ^ Berger, Dan. "Why does Tungsten not 'Kick' up an electron from the s sublevel ?" . Bluffton College, USA. ^ 4.0 4.1 4.2 Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements . Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9 ^ Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics Boca Raton, Florida : CRC Press . p. 6-134. ISBN 978-1-4200-9084-0 ^ Tolias P. (2017). "Analytical expressions for thermophysical properties of solid and liquid tungsten relevant for fusion applications". Nuclear Materials and Energy . 13 : 42–57. arXiv :1703.06302 Bibcode :2017arXiv170306302T . doi :10.1016/j.nme.2017.08.002 . S2CID 99610871 . ^ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds" (PDF) . CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 978-0-8493-0486-6 the original (PDF) on 2011-03-03. ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics . Boca Raton, Florida: Chemical Rubber Company Publishing. p. E110. ISBN 978-0-8493-0464-4 ^ "Tungsten" . Royal Society of Chemistry . Royal Society of Chemistry . Retrieved May 2, 2020 .^ van der Krogt, Peter. "Wolframium Wolfram Tungsten" . Elementymology& Elements Multidict. Archived from the original on 2010-01-23. Retrieved 2010-03-11 . Lua error in mw.title.lua at line 229: too many expensive function calls.